News
OSCAR is an online ML-powered tool for organoid cell counting using bright-field images
December 3rd, 2025
Our new article has just been published in Cell Reports Methods. In it, we introduce OSCAR, a workflow that fills an important gap in organoid research by estimating the number of cells in an organoid culture directly from bright-field images. Existing tools can count organoids and measure their morphology, but they do not provide reliable estimates of total cell numbers, which are essential for applications such as co-culture experiments. OSCAR combines a Mask R-CNN segmentation model to identify organoids and measure their area with an empirical regression approach that relates organoid area to cell number. In validation tests, OSCAR estimated well-level cell counts within ±16 percent of the true value. As an online tool, OSCAR provides fast, accessible and robust quantification to improve the consistency of organoid-based assays.
Read the full paper here
We are delighted to announce that our team has been awarded a Cancer Research UK Discovery Programme Award for our project “Optimisation of anti-cancer CD4+ T cell responses through molecularly-informed modification of HLA class II antigens.”
Read more about the Discovery Programme Awards here
Friday 5th September, 2025

“Every day I get to discover something that nobody in the world has ever known before.” - Dr Mathew Clement
As part of hashtag#ChildhoodCancerAwarenessMonth, Arthur spoke with our future leader, Dr Mathew Clement, to learn about his job as a researcher - aiming to discover potential new medicines to treat brain tumours.
Arthur, 10, was diagnosed with a brain tumour at just two years old. Following surgeries in 2017 and 2018, Arthur had left-sided weakness and learnt to walk again. Last year, he embarked on a mammoth challenge – to conquer enough British peaks to equal the height of Mount Everest – an astonishing 8,849 metres.
Arthur completed his challenge in November 2024 and smashed his fundraising target, which includes matched funding by his Dad Henry’s employer, Macquarie, bringing the total to over £54,000!
You can watch Arthur and Mathew’s full conversation on our YouTube Channel, linked below!
https://bit.ly/46qSaXo
Friday 5th September, 2025

Congratulations to ImmunoServ for winning the Welsh Government St David's Award recognising their contributions to Innovation, science and technology.
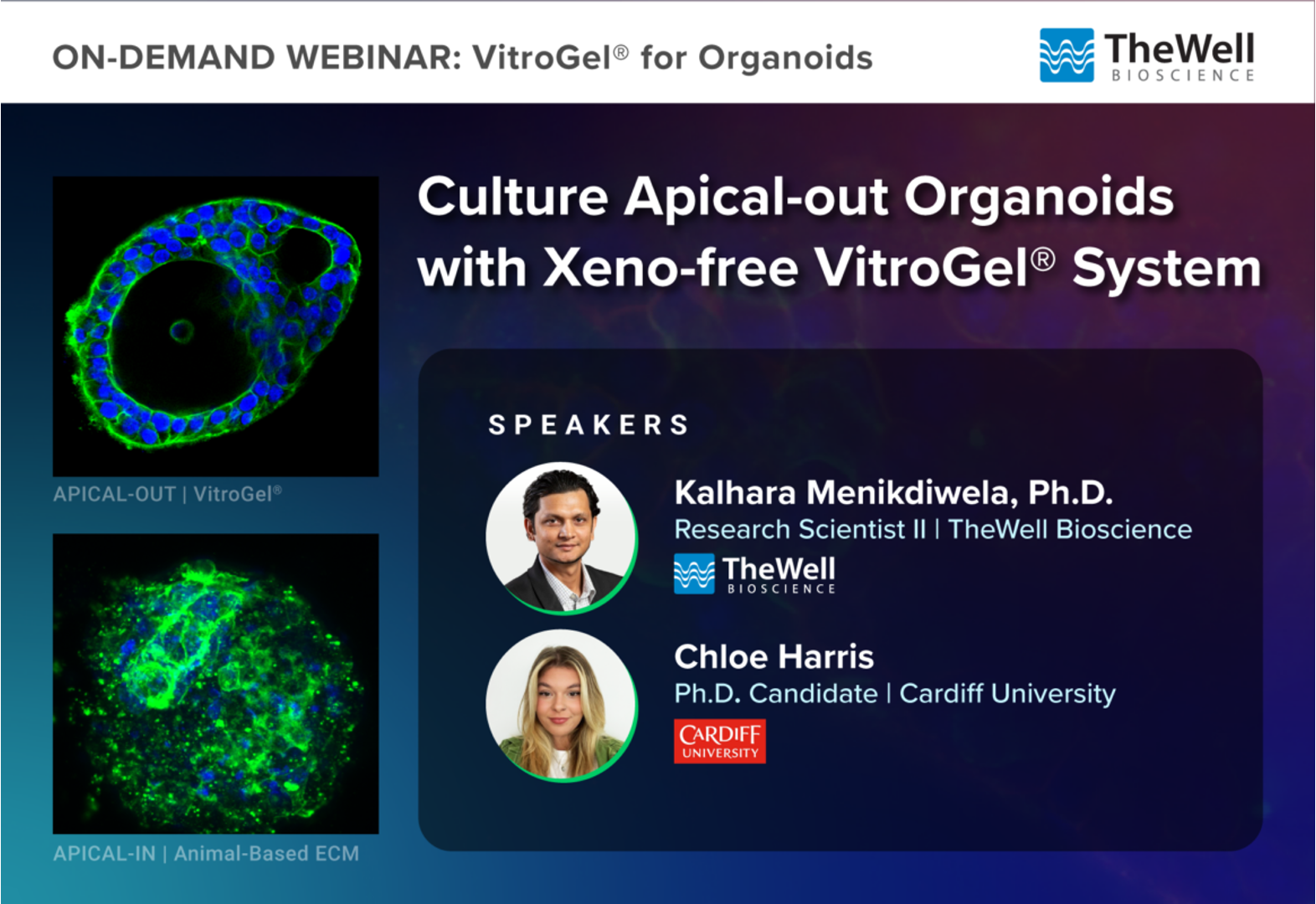
Friday 28th March, 2025

As part of my ongoing research into animal-free alternatives for Matrigel in organoid culture, I discovered that one synthetic gel, VitroGel, induced a unique morphological change to these organoids: causing them to flip inside out (apical out)! I had the pleasure of presenting these findings on the attached webinar, in collaboration with TheWell Biosciences, where I share more about these findings and the rationale behind my project. - Chloe Harris NC3Rs-funded PhD student.
Watch the webinar on this link
March 17th September, 2025
A targeted single mutation in influenza A virus universal epitope transforms immunogenicity and protective immunity via CD4+ T cell activation
June 6th, 2024
New study on targeting a single mutation in influenza A virus universal epitope transforms immunogencity and protective immunity led by James Geary, Dr Sarah Curtis, and Dr Bruce MacLachlan is now published online in Cell Reports!
CD4+ T cells are central to adaptive immunity. Their role in cross-protection in viral infections such as
influenza and severe acute respiratory syndrome (SARS) is well documented; however, molecular rules
governing T cell receptor (TCR) engagement of peptide-human leukocyte antigen (pHLA) class II are
less understood. Here, we exploit an aspect of HLA class II presentation, the peptide-flanking residues
(PFRs), to ‘‘tune’’ CD4+ T cell responses within an in vivo model system of influenza. Using a recombinant
virus containing targeted substitutions at immunodominant HLA-DR1 epitopes, we demonstrate limited
weight loss and improved clinical scores after heterosubtypic re-challenge. We observe enhanced protection linked to lung-derived influenza-specific CD4+ and CD8+ T cells prior to re-infection. Structural
analysis of the ternary TCR:pHLA complex identifies that flanking amino acids influence side chains in
the core 9-mer peptide, increasing TCR affinity. Augmentation of CD4+ T cell immunity is achievable
with a single mutation, representing a strategy to enhance adaptive immunity that is decoupled from vaccine modality.
The full paper can be accessed here
Structural definition of HLA class II-presented SARS-CoV-2 epitopes reveals a mechanism to escape pre-existing CD4+ T cell immunity
July 19, 2023
New study on CD4+ T cell epitopes of SARS-CoV-2 led by Dr Bruce MacLachlan is now published online in Cell Reports!
We define model antigens presented by HLA-DR1 and assess the impact of Spike mutations on T cell recognition. We aimed to provide high resolution epitope information of emerging CD4+ T cell epitopes and thus solved x-ray crystallographic structures of six peptide-HLA-DR1 complexes; 3 Spike and 3 non-Spike (M, nsp3 & nsp14). We could solve complexes of high and low affinity binding peptides and could not correlate peptide affinity with immunogenicity.
As the pandemic unfolded, we observed Omicron mutations in two of the Spike epitopes we described structurally. Testing in HLA-DR1+ donors who had been vaccinated, we observed that donors could no longer recognise BA.1 Omicron variant forms.
To understand this we solved further structures of Omicron variant peptide-HLA-DR1 complexes: observing a single amino acid mutation was sufficient to induce a drastic change in a presented epitope through inducing an epitope "register shift”. This effectively creating a "new" epitope to the perspective of T cells.
This shows that CD4+ T cell memory is finely poised at the level of antigen presentation which is unique to the "open ended" nature of the HLA-II groove.
The full paper can be accessed here
Successful Immunotherapy is Linked to Devlopment of Specialised Blood Vessels in Solid Cancers
December 15th 2022
Applying three-dimensional imaging methods, researcher, Dr Stefan Milutinovic, showed that cancers which are successfully killed by the immune system contain networks of blood vessels which are specialised in their ability to allow immune cells to enter tumours. The work has just been published in Cancer Research Communications.
Prof Awen Gallimore said that the new technology offers the possibility to better understand the co-operation between blood vessels and immune activation and moreover, that the results indicate that targeting blood vessels has the potential to significantly improve the success of the current immunotherapies.
The lab, which is funded by Cancer Research UK, is currently building on this work to find ways of inducing development of these blood vessels in solid cancers.
Read the full paper here.