Patient-Derived Colorectal Organoid BioBank
Generated by Dr Stephanie Burnell
BurnellS@Cardiff.ac.uk,
Stephanie Burnell | LinkedIn
Organoids are miniature, three-dimensional structures grown from stem cells that mimic the architecture and functionality of real organs. These complex models are invaluable for research as they provide a more accurate representation of human biology when compared to traditional cell cultures. Organoids are particularly useful in studying disease mechanisms, drug responses, and personalised medicine approaches. Visually, organoids resemble tiny, self-organizing clusters of cells, each unique to the tissue they represent.
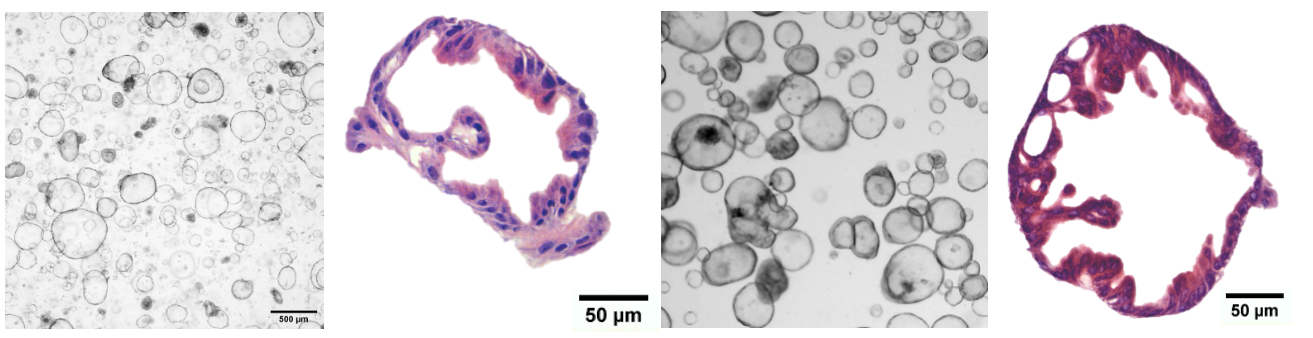
In our lab, the organoids we have developed have been derived from patient tissue, kindly donated by patients undergoing surgery. We have successfully developed colorectal organoids from both healthy and tumour tissue, which replicate the heterogeneity found in patients. These organoids serve as powerful tools for advancing our understanding of colorectal cancer (CRC), developing effective treatments and for exploration of how the immune system might recognise, isolate and kill malignant but not healthy cells.
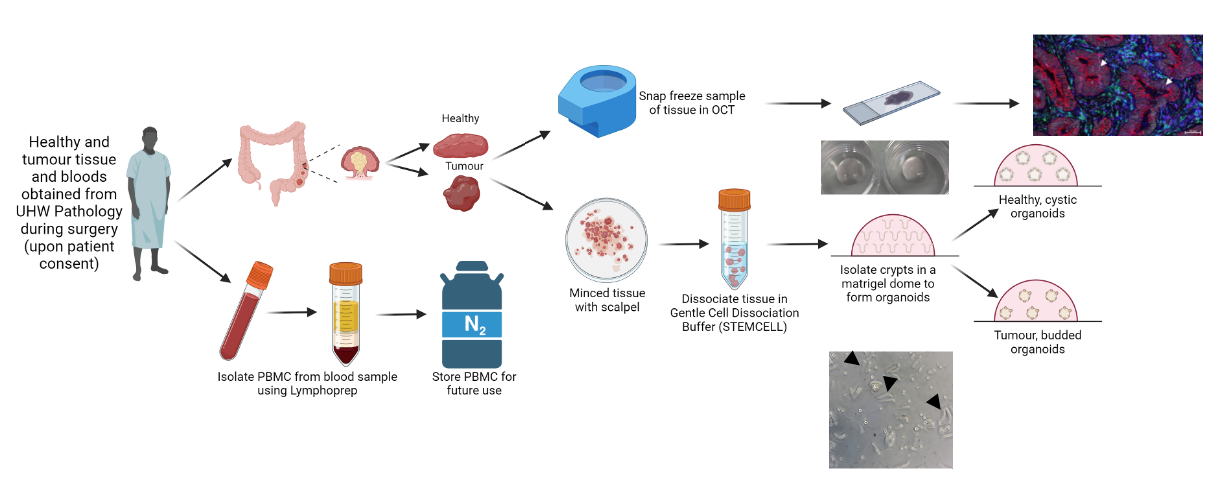
Generation of a colorectal organoid biobank. Following informed consent, tissue and blood samples are obtained from patients undergoing surgery and transported to the lab for processing. From the blood sample, peripheral blood mononuclear cells are isolated and frozen in liquid nitrogen for future use. The tissue samples are split – half is frozen in OCT and stored at -80°C for future staining and comparison to original tissue and the other half is dissociated and processed to isolate the colonic crypts. The crypts are then embedded in a matrigel scaffold to encourage the formation of organoids.
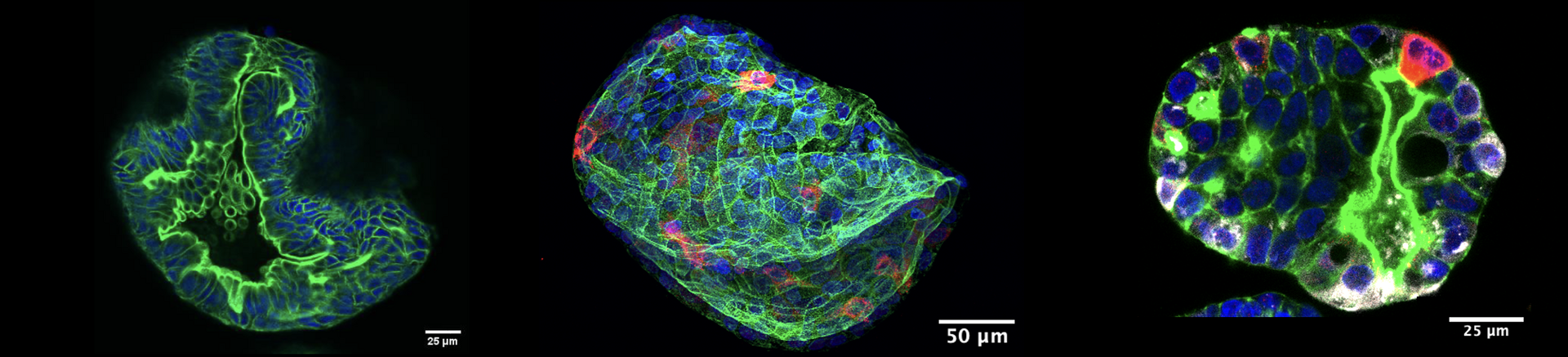
Characterisation of Colorectal Organoids
Dr Stephanie Burnell and Chloe Harris
BurnellS@Cardiff.ac.uk, HarrisC27@cardiff.ac.uk
We are characterising our bank of colorectal organoids to determine the mutational load, HLA status, and morphology and prove that the organoids contain multiple cell types. We are doing this via various methods including whole genome and RNA sequencing (WGS. RNAseq), flow cytometry and confocal microscopy. Stay tuned for updates as we carry out our characterisation experiments. This information, alongside clinical information, will provide researchers with a more specific and clearer picture of the model they are working with. Additionally, we are developing a computational pipeline to estimate the number of cells within an organoid using only a brightfield image, our manuscript is in preparation.
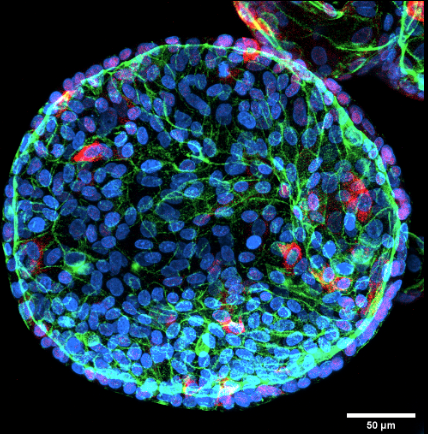
Fluorescent staining of colorectal organoids. Our organoids have been stained for lysozyme (white) and mucin (red) to demonstrate the presence of paneth and goblet cells respectively. The presence of these cells demonstrates the existence of differentiated cells within the organoid and confirms that it is a multi-cellular model system.
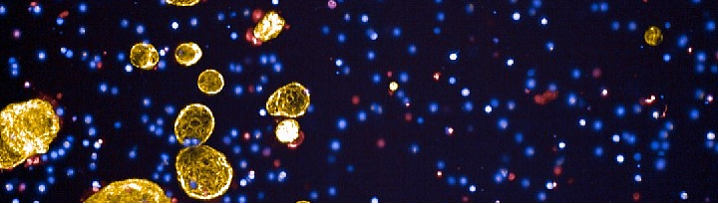
Organoid/T Cell Co-culture Model
Dr Stephanie Burnell, Chloe Harris, Lorenzo Capitani and Dr Yuan Chen
BurnellS@Cardiff.ac.uk, HarrisC27@cardiff.ac.uk, CapitaniL@cardiff.ac.uk, ChenY158@cardiff.ac.uk
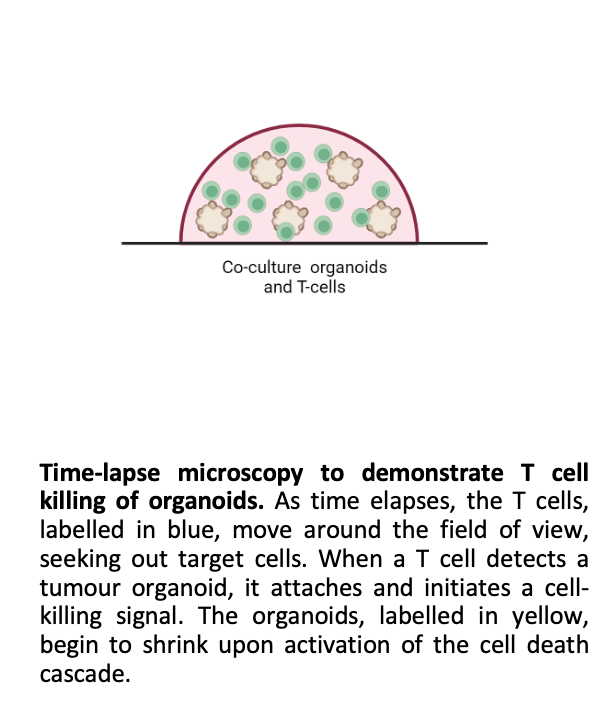
Our co-culture model involves growing CRC organoids alongside T cells, creating a dynamic system to study immune interactions and test immunotherapies. Using the Opera Phenix high-content imaging system, we have captured detailed images showcasing the interactions between T cells and organoids. Co-culture experiments demonstrate effective T-cell infiltration and tumour-cell killing. We invite researchers and clinicians to collaborate with us in utilising this co-culture system to test novel therapies and investigate tumour-immune dynamics.

Funding from this project has been received from Wales Cancer Research Centre, Wales Cancer BioBank, NC3Rs, GW4, UKRI Harmonised Impact Acceleration Account, Institutional Strategic Support Fund Public Engagement Award, Future Leaders in Cancer Research Seedcorn Funding.